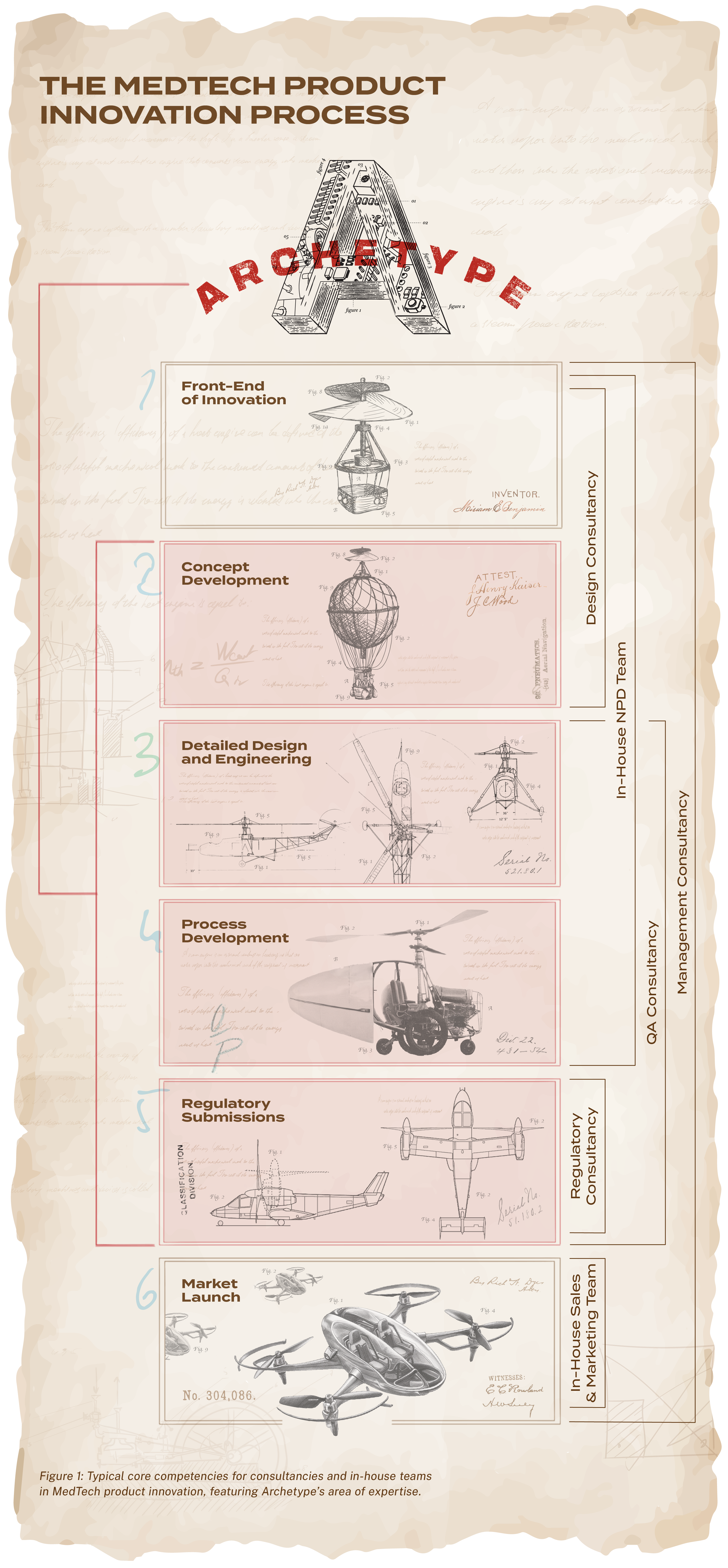
The Blueprint
FOR MEDTECH SUCCESS
Archetype is an innovation management consultancy with unparalleled expertise in obtaining market approval for medical devices.
Our services include:
OPTIMISE
Thoroughly examine and streamline your product innovation practices to optimise for market approval
CUSTOMISE
Fully customised innovation procedures that help you make the right moves at the right time for efficient market approval.
ASSESS
An investor-ready deep-dive technical evaluation of your chances for market approval.
EDUCATE
Train your team to be a force multiplier that is mindful of market approval at every step of the innovation process
WHO WE ARE
Our Medtech Manifesto
In the MedTech space today, there is no blueprint for success. A constantly changing market creates difficulties at every turn, and it’s never been harder to make something new. Even legacy products now face an uncertain future.
In this environment, the already all-consuming question, “Can it be done?” becomes, “Even if it can be done, will it be allowed?”
At the same time, the rewards for success have never been greater. Not just in terms of money, but in terms of the number lives around the world that can be improved.
That’s what motivates us. We consider our purpose profound: to grant patients access to the transformative power of cutting-edge medical devices. We seek to empower MedTech entrepreneurs with strategic insights, unwavering support, and the guidance needed to achieve market approval.
Because it is only when your product is both functional AND available that it can fulfill its purpose: helping people.
That’s why we made our company.
Archetype. Making MedTech Success.
Our Founder
Meet Stuart Grant, the driving force behind Archetype. Few individuals in the MedTech sector can claim the innovation, product design, engineering, regulatory, and project management expertise required to take complex Medical Devices to market. Stuart is one of them.
Renowned for his role as a fixer, challenger, and change-maker, highlights of Stuart’s 25-year career include spearheading groundbreaking initiatives at DePuy Synthes and Johnson & Johnson MedTech.
An inventor and tinkerer at heart, Stuart is the named inventor on multiple patents for market-leading medical devices, a Chartered Engineer of the Institute of Mechanical Engineers, and has a PhD in Technology and Innovation Management.
Read MORE
Our Founder

Stuart Grant
BSc (HONS), MSc, PhD, CEng
Founder/
Principal Consultant
Our Network is Your Blueprint


During his 25 years at MedTech giant Johnson & Johnson, Stuart built an unparalleled network that spans continents and specialties.
He has worked directly in the UK, US, Asia-Pacific, and India and worked closely with MedTech counterparts in key European markets like Germany, France, Italy, Switzerland, and Ireland.
His trusted contacts are pioneers and experts in groundbreaking fields like AI and robotics, SaMD, 3D printing, VR and AR, neurotech, health wearables, and even gaming tech.
The Archetype Network grants startups and scale-ups an invaluable gateway to the world’s first-class MedTech minds. As a consultant on your project, Stuart will curate the perfect team to match your needs while he remains at the helm, overseeing every facet of the work.
Get in touch to learn how you can tap into our network. And if you’d like to become part of the network, we’d love to hear from you, too.
What we do
Our services
Optimise
Thoroughly examine your product innovation practices and find ways to streamline and optimise. This will be no surface-level inspection followed by a PowerPoint presentation. This is a nuts-and-bolts strategic review by one of the best in the business. Inefficiencies and pain points will have nowhere to hide.
Areas of focus may include:
- Product Innovation Strategy
- Regulatory Pathway Design
- Risk Appraisal & Mitigation Strategy
- Processes & Systems Design
- Programme Management
Customise
While there is no blueprint for MedTech success, there is also no need to start from nothing. We’ve picked up a few tricks in the trenches, so we can offer a fully customised suite of innovation procedures that will help you make the right moves at the right time.
Tailored solutions include:
- Design Control Procedures
- Requirements Management
- Risk Management
- Regulatory Assessment
Assess
Use our technical expertise to gauge the state of the market you’re targeting and provide an honest evaluation of your chances for market approval. To have the best chance of success, you need to know exactly what you’re up against.
Areas of expertise include:
- Pre-investor validation reporting
- Investor due diligence reporting
- Investor status report
Educate
The most valuable asset you can hope for is an in-house team that is perpetually mindful of market approval. A well-trained team is a force multiplier that can make the difference between success or failure. We are ready and willing to train your team on any number of topics so that they can minimise surprises and maximise productivity.
Training opportunities include:
- Medical Device Innovation Processes
- Fundamentals of Innovation
- MedTech 101s
- Workshop facilitation
ARE YOU READY FOR THE MARKET?
ARE YOU READY FOR THE MARKET?
If getting market approval for a MedTech device sounds like rocket science, may we suggest MARS?
Take Archetype’s proprietary Market Approval Readiness Survey (MARS) to get a high-level assessment of the likelihood that you will receive market approval for your device.
Built on Principal Consultant Stuart Grant’s decades of experience in the MedTech industry, Stuart will walk you through the Market Approval Readiness Survey one-to-one, and quickly follow-up with a fully customised report that rates your readiness for market approval and provides actionable insights on areas for improvement.
Take me to MARS
A mutual NDA will be provided as part of the MARS process.

How we work
It seems like there’s already a consultant for every need in the MedTech product innovation process. So where does Archetype fit? In a word: everywhere.
We use our rare combination of knowledge, skill and experience to integrate seamlessly with your existing consultants and in-house teams from concept to launch, operating with the specific purpose of keeping market approval top-of-mind while working together toward the ultimate goal – bringing a world-changing MedTech product to market.

“Stuart brings significant real-world experience and a thorough and systematic approach to problem solving to the table.”

“Stuart has a wealth of experience in the orthopaedics industry that couples well with his process-oriented mindset.”

“Stuart not only provides clients with his engineering talent, expertise in Product Realization and NPD, but they also benefit from his drive and can-do attitude.”
Our SUCCESS
This series of brief success stories highlights some of our proudest moments and biggest wins - offering a glimpse into the type of market approval mastery we bring to the table.
Avoiding Frankenstein’s Monster While Streamlining NPD
Faced with harmonising NPD across business units, learn how streamlined processes improved collaboration, decision-making, resource allocation, and efficiency.
Read StoryDancing with the MDR
The EU MDR has sent shockwaves through the MedTech industry. Read more about the complexities to recertifying legacy devices whilst future-proofing innovation.
READ STORYDecomposing a Design Requirement Symphony
Navigating standards is crucial for developing medical devices. Read on for innovative solutions that translate standards into clear design requirements.
Read StoryWhat we think
Explore our perspectives on MedTech’s pivotal developments. Legislation, regulation, industry trajectories, compelling case studies, we weigh in on MedTech’s critical issues and opportunities with insights distilled from decades of front-line experience.

Why integrate RTM and RM?
Dr. Stuart Grant, Principal Consultant at Archetype Medtech, explains how integrating RTM and RM facilitates regulatory approval.
As early as possible: The timing dilemma for the medtech innovator
This piece examines the tradeoffs associated with starting development too early and how to reconcile that desire with reality.
Alternate FEI frameworks
FEI frameworks are not ‘one-size-fits-all’. This article looks at different FEI models most suited to the healthcare industry

Essential Reading 2
This piece examines the concept of a ‘Minimum Viable Product’ and looks at how we can achieve the ‘Threshold of Good’ in the high stakes world of MedTech.
PRODUCT INNOVATION IN MEDTECH:
THE HIDDEN PITFALLS TO MARKET APPROVAL
Read this guide to spotting – and solving – the hidden problems that can put market approval for your MedTech device in doubt.
Navigating the complex MedTech approval process requires more than just innovation. Our white paper, "The Hidden Pitfalls to Market Approval", explores both well-known and overlooked challenges that can stop projects in their tracks. Learn how to:
- Address regulatory standards
- Manage risk
- Optimise design for user safety
Read now to stay ahead of potential delays and secure your device's successful market entry.
Get in touch
If you want to know more about how Archetype can help get your medical device market approved, we would be delighted to hear from you. Complete the form below and we will be in touch at the speed of innovation.